Breath of life: This medical tech startup is healing newborns with asphyxia through a cooling device
Bengaluru-based Sensivision Health Technologies strives to save the lives of newborns and minimise the risk of brain damage due to birth asphyxia using a therapeutic cooling device—which cools the baby, slows down metabolism, and gives the brain time to heal.
The neonatal intensive care unit (NICU) is a zone filled with high stakes and emergency situations, where every second counts. Dr Raghuvamsi Chaitra, a neonatologist at Starkids Children’s Hospital in Anantapura, Andhra Pradesh, is no stranger to such situations.
He recalls an incident a few months ago when a newborn baby with perinatal asphyxia was brought into the NICU along with the mother.
Perinatal asphyxia or birth asphyxia is a condition in which a newborn’s brain doesn’t receive enough oxygen at childbirth. “There were complications, and the baby did not cry at birth,” says the doctor.
Dr Chaitra and his team swung into action immediately to resuscitate the baby.
The baby was limp and blue, which meant it needed immediate cooling therapy to prevent the risk of brain damage.
The common practice is to employ manual cooling using ice packs and manually monitoring the temperature and adjusting the cooling as needed. The challenge with this method is that the ice melts, resulting in fluctuating temperatures. Besides, the ice packs have to be constantly replaced manually.
To avoid these complexities and maintain a constant temperature, Dr Chaitra used REVIVE, a medical device developed by Sensivision Health Technologies, which adjusts the temperature automatically without the need for manual intervention.
After three days of cooling and eight hours of rewarming, the baby was fed and discharged on the sixth day. Scans and follow-up checkups showed that the baby was doing fine.
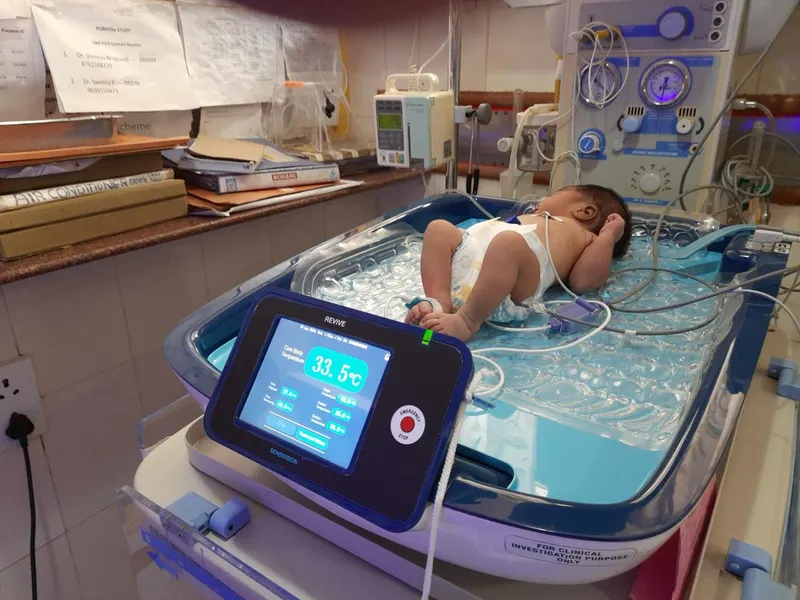
Dealing with birth asphyxia
Hypoxic-ischemic encephalopathy (HIE) or perinatal asphyxia causes about 9 lakh deaths every year, according to the World Health Organization. Studies reveal that 2-16% of newborns in India are affected by it, and the fatality rate ranges from 38.5 to 74%.
The condition can also cause permanent damage to the brain, resulting in conditions such as cerebral palsy and other mental developmental disorders.
According to medical practitioners, when there isn’t sufficient oxygen, the baby’s body temperature is lowered, within six hours of birth, to slow down metabolism and other bodily functions. The need for oxygen and energy goes down, thus giving the brain time to recover from the trauma caused due to lack of oxygen.
“Once you cool the baby, the risk of further brain injuries or secondary brain injuries comes down. And the babies have less chance of having neurological problems later in their life,” explains Dr Chaitra.
This is what Bengaluru-based healthtech startup Sensivision Health Technologies does with REVIVE, a patented therapeutic hypothermia device device.
“The normal temperature is about 37 degrees Celsius. We bring down the baby's temperature to 33.5 degrees (using REVIVE) and maintain it for three days. After 72 hours, we slowly warm the baby again,” explains Jayadeep Unni, Founder, Sensivision, and an industry veteran with nearly 27 years of experience in biomedical engineering.
Founded in 2015, Sensivision began selling REVIVE about a year ago—after nearly a decade of research and development.
What REVIVE brings to neonatal care
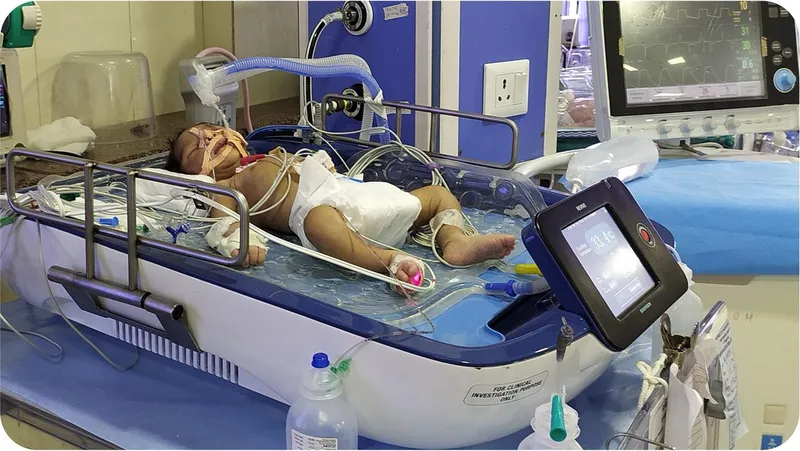
REVIVE provides whole-body cooling and warming and comes with integrated technology that monitors the baby’s response to the cooling, its cerebral function, and the seizure activity of the brain.
Unni claims REVIVE is the only one of its kind that can monitor seizures in a baby.
REVIVE is a servo-controlled device, which maintains constant temperature. Servo control is a technology that enables precise control of the device through a feedback mechanism.
As the device is fully automated, it reduces the need for constant monitoring or intervention from nurses and clinicians. This is a huge relief to hospitals where staff shortage, especially in neonatal care, is a pressing concern, says Unni.
The medical device can be operated by trained doctors and nurses in the NICU. It can also be used during transit when the baby is moved in an ambulance to another hospital.
Sensivision has installed REVIVE in 20 hospitals in India—both government and private institutions—across Tier I, II and III cities, including PGI Chandigarh, Wadia Children’s Hospital and Hinduja Hospital in Mumbai, and Niloufer Hospital in Andhra Pradesh. The device is said to have saved the lives of around 250 newborns.
“Smaller cities and villages are where complications occur more due to lack of follow-up with the mother during pregnancy,” says Unni.
Development and challenges along the way
Doctors from St John’s Hospital, Bengaluru, and Starkids Children’s Hospital in Anantapura, among others, were engaged with Sensivision right from the early stages of development of REVIVE.
“When medical devices are built, they come from these big companies and get pushed into the market, and a hospital or a doctor is forced to use them. Their input into designing solutions is not too much. So, engaging with doctors gave us a good insight into the typical challenges that neonatologists face while treating HIE condition,” says Unni.

The Sensivision team
The startup also partnered with hospitals including JJM Medical College in Davangere, Karnataka, and S Nijalingappa Medical College in Bagalkot, Karnataka, for clinical trials.
As per regulatory compliance requirements, medical devices have to meet certain electrical, mechanical, and clinical safety standards according to their intended use and risk classification.
Based on the risk level to the patient, medical devices in India are categorised from ‘low risk’ (Class A) to ‘highest risk’ (Class D) by the Drug Controller General of India (DCGI).
Lack of clarity pertaining to the classification was a significant challenge during the development of REVIVE, says Unni.
“While we were in the advanced stages of product development, the classification (of REVIVE) was still a work in progress. We had to expend a lot of time and effort before we could get clarity on our classification. This involved a lot of back-and-forth with the DCGI office. Finally, our device ended up being classified as Class B,” explains the founder.
Class B devices come under the low-to-moderate risk category; they are non-invasive but come in contact with intact mucous membranes.
Availability of labs with the right infrastructure for thorough testing to meet compliance requirements was another challenge that Sensivision faced. Convincing government hospitals that what REVIVE does can save lives is also not easy, adds Unni.
Funding and future
Though Unni does not disclose the price of the device, he says REVIVE offers significant cost benefits.
“For the features we have built into this product, a hospital will have to get two separate devices costing at least three times what our product costs. And, further, these would have to be imported.”
Sensivision has been funded by Biotechnology Industry Research Assistance Council, Venture Center (an incubator in Pune), C-CAMP ( Centre for Cellular and Molecular Platforms, Bengaluru), and Elevate Incubation Programme, an initiative of the Karnataka Government.

REVIVE being demonstrated to Prime Minister Narendra Modi at Biotech Startup Expo in Delhi in June 2022
Social Alpha (Tata Trust), Selco Foundation, PATH Foundation, and Millennium Alliance have also funded the startup.
The company is actively looking to raise around Rs 10 crore to export to the markets of Africa, Sri Lanka, Nepal, Bhutan, Bangladesh and the Middle East.
Sensivision aims to clock a revenue of Rs 4 crore in FY25 and touch Rs 20 crore-30 crore in the next few years.
US-based Masimo and Belmont Medical Technologies are some of the other healthtech players offering therapeutic cooling devices in India.
Sensivision plans to launch its second product, Illuminatus, within a year. This device monitors the blood oxygen levels in the brain tissue and the brain’s electrical activity in babies.
Unni believes every life is worth fighting for and seeks to help medical practitioners save more lives and bring hope to families.
Edited by Swetha Kannan








